Abstract
Background
Patients (Pts) with multiple myeloma (MM) experience prolonged immunosuppression due to the incurable nature of the disease and corresponding treatment modalities. Due to this many MM pts with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) require hospitalization, with an increased mortality rate over healthy adults. Two mRNA vaccines against (SARS-CoV-2): BNT162b2 & mRNA-1273 were approved under an emergency use authorization (EUA) by the Food and Drug Administration (FDA) due to the high efficacy in preventing SARS-CoV-2. The aim of this study was to analyze the antibody (Abs) response in all pts with plasma cell disorders (PCD) including MM, AL-Amyloidosis, and smoldering myeloma (SMM) who are on active treatment.
Patients & Methods
All pts (MM, AL-Amyloidosis, and SMM) on active treatment who received SARS-CoV-2 mRNA vaccine were identified at the University of Kansas Health System between January 2021 to July 2021and reviewed retrospectively. Descriptive analyses were performed on available data for patient characteristics. Abs against SARS-CoV-2 were measured using methodology approved by the FDA (enzyme-linked immunosorbent assay; cPass SARS-CoV-2 Neutralizing Antibody Detection Kit; GenScript, Piscataway, NJ). We stratified pts into clinically relevant responders (>250 IU/mL), partial responders (50-250 IU/mL), and non-responders (<50 IU/mL)
Results
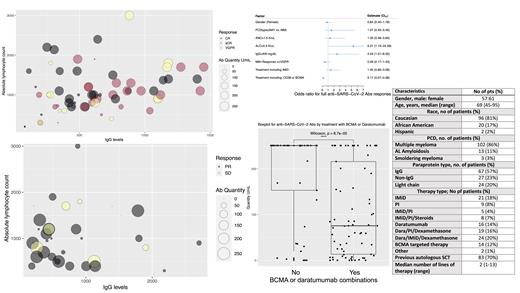
A total of 118 pts were identified in our analysis and are described in Table 1. Of the total pts, 102 (86%) had MM, 13 (11%) pts had AL-Amyloidosis, and 3 (3%) pts had SMM. Median age was 69 years (45-95), 96 pts (81%) were Caucasian, and 57 (48%) were male. Median lines of prior treatment was 2 (1-13). Active PCD patients were treated with single-agent therapy in 60 pts (51%), doublet-based therapy in 5 pts (4%), and triplet-based therapy in 51 pts (43%). Daratumumab based therapy was utilized in 59 pts (50%). All pts included received two doses of either BNT162b2 or mRNA-1273. At the time of abs testing 82 patients (69%) were in a very good partial response (VGPR) or better, 29 pts (25%) were in partial response, while 7 pts (6%) had stable disease. Five pts (4%) had COVID-19 infection prior to the vaccine. The median time between thesecond dose of the vaccine and testing for Abs was 100 days (34-190). Only 46 pts (39%) developed an adequate response, 36 pts (30.5%) had a partial response, while 36 (30.5%) did not respond to the vaccine. Low Ab levels were seen in all PCD subtypes with the following mean levels: SMM :25.4 (5.4- 36.9) IU/mL, MM 148 (0- >250) IU/mL, and AL- Amyloidosis 92.35 (range 0- >250) IU/mL. Among the 5 pts with COVID-19 infection prior to the vaccination, full Abs response was observed in 4 pts, and 1 patient had no Abs response. Type of treatment did not affect the response to treatment in any clinically meaningful way. The odds ratio of achieving a clinically relevant Abs response was higher in pts with absolute lymphocyte counts>0.5 K/uL (p=0.01) and IgG levels> 400 mg/dL (p=0.04) and lower in pts receiving treatments with daratumumab combinations or anti-BCMA therapy (p<0.0001). Higher levels of anti-SARS-CoV-2 Abs were observed in pts with ≥ VGPR (mean≈147 IU/mL) compared to <VGPR (mean≈ 119 IU/mL). However, in this dataset, this difference was not statistically significant (p=0.17).
Conclusion
mRNA vaccine Ab response is lower in PCD pts getting active treatment compared with the general population. For PCD patients on active treatment, mRNA vaccine produced full antibody responses and partial responses in 39% and 30.5% of pts, respectively. anti-SARS-CoV-2 abs are especially low for patients on daratumumab combinations or anti-BCMA therapy, low lymphocytes, and low IgG levels at the time of vaccination. Some PCD may not develop anti-SARS-CoV-2 abs despite vaccination and/or previous COVID-19 infection. Therefore, checking anti-SARS-CoV-2 abs may be clinically useful in identifying patient's response. Further prospective studies should ascertain the value of a 3 rd vaccine dose in this population.
Mahmoudjafari: Omeros: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees. McGuirk: Astelllas Pharma: Research Funding; Juno Therapeutics: Consultancy, Honoraria, Research Funding; EcoR1 Capital: Consultancy; Gamida Cell: Research Funding; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Fresenius Biotech: Research Funding; Bellicum Pharmaceuticals: Research Funding; Novartis: Research Funding; Pluristem Therapeutics: Research Funding; Allovir: Consultancy, Honoraria, Research Funding; Kite/ Gilead: Consultancy, Honoraria, Other: travel accommodations, expense, Kite a Gilead company, Research Funding, Speakers Bureau; Novartis: Research Funding. Atrash: Jansen: Research Funding, Speakers Bureau; AMGEN: Research Funding; GSK: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal